abacavir
12 May 2018. Related: ARVs and PrEP, Nukes


12 May 2018. Related: ARVs and PrEP, Nukes

12 April 2016. Related: ARVs and PrEP, PIs
![]()
2 February 2025. Related: ARVs and PrEP, Fixed dose combinations, NNRTIs, Nukes

Generic versions of Atripla (pictured) will look different. It is sometimes called TLE or AF2B.
Generic versions include Atenef, Atreslawin, Atroiza, Citenvir, Heftenam, Odimune, Rizene, Teevir, Trenvir, Tribuss, Triolar, Trivenz, Truno, Trustiva, Viraday and Vonavir.
Full details about Atripla / TLE (efavirenz + tenofovir DF + emtricitabine/lamivudine)
1 April 2013. Related: ARVs and PrEP, Nukes
![]()
1 January 2025. Related: ARVs and PrEP, Integrase inhibitors
no separate formulation
30 August 2024. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes

Biktarvy is a pink/grey film-coated tablet (15 mm x 8 mm) with “GSI” on one side and “9883” on the other.
1 October 2025. Related: ARVs and PrEP, Integrase inhibitors, NNRTIs
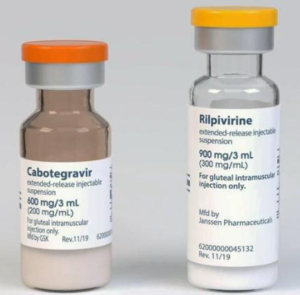
(not to scale)
Cabotegravir-LA and rilpivirine-LA are long acting injections given by intramuscular injections every two months.
Full details about cabotegravir LA + rilpivirine LA (Vocabria+Rekambys or Cabenuva)
27 October 2025. Related: ARVs and PrEP, Integrase inhibitors, PrEP
Cabotegravir-LA (CAB-LA) is a long acting injection given by intramuscular injections every two months.
12 April 2016. Related: ARVs and PrEP, PK booster
![]()
26 August 2012. Related: ARVs and PrEP, Nukes
![]()
4 January 2016. Related: ARVs and PrEP, Nukes
![]()
No longer recommended in the UK.
30 June 2023. Related: ARVs and PrEP, PIs

800 mg formulation shown
4 January 2016. Related: ARVs and PrEP, Nukes
![]()
No longer recommended in the UK.
12 May 2018. Related: ARVs and PrEP, Nukes, PrEP
![]()
![]()
10/200 mg (white) and 25/200 mg (blue)
30 August 2024. Related: ARVs and PrEP, Integrase inhibitors
![]()
Dolutegravir 50 mg is a 9 mm diameter round yellow tablet.
18 November 2018. Related: ARVs and PrEP, NNRTIs
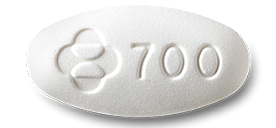
Doravirine is a white tablet embossed with “700” on one side. (Source: aidsinfo.nih)
18 September 2019. Related: ARVs and PrEP, Fixed dose combinations, NNRTIs, Nukes

Delstrigo is a light yellow tablet embossed with “776” on one side. (Source: aidsinfo.nih)
30 August 2024. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes
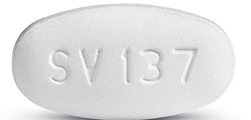
Dovato is a white tablet embossed with “SV137”. (Source: aidsinfo.nih)
1 September 2016. Related: ARVs and PrEP, NNRTIs
![]()
1 May 2018. Related: ARVs and PrEP, Integrase inhibitors

Single drug formulations of elvitegravir are no longer used. Instead, elvitegravir is included with other drugs in fixed dose combinations.
12 April 2016. Related: ARVs and PrEP, Nukes
![]()
14 May 2017. Related: ARVs and PrEP, NNRTIs
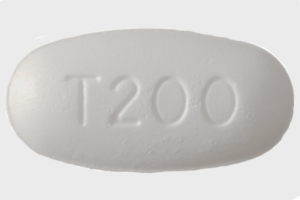
(200 mg shown)
3 May 2013. Related: ARVs and PrEP, Fixed dose combinations, NNRTIs, Nukes

12 October 2016. Related: ARVs and PrEP, PIs, PK booster
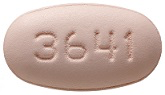
26 August 2012. Related: ARVs and PrEP, PIs
![]()
1 February 2021. Related: ARVs and PrEP, Entry inhibitors
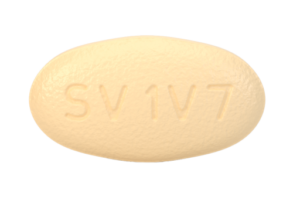
30 August 2024. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, NNRTIs, Nukes, PIs
A generic drug is one that is manufactured by a different pharmaceutical company to the company that invented the medicine.
12 April 2016. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes, PK booster
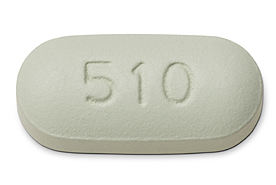
Full details about Genvoya: elvitegravir + cobicistat + emtricitabine + TAF (E/C/F/TAF)
22 March 2023. Related: ARVs and PrEP, Entry inhibitors, monoclonal antibody (mAb)
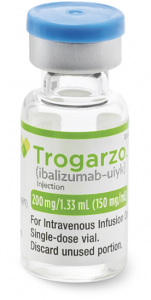
Ibalizumab is a 200 mg concentrate that is then made up as an infusion.
26 August 2012. Related: ARVs and PrEP, PIs
![]()
No longer recommended in the UK.
12 May 2018. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, NNRTIs
![]()
12 May 2018. Related: ARVs and PrEP, PIs
![]()
No longer recommended in the UK.
2 January 2018. Related: ARVs and PrEP, Nukes
![]()
4 January 2022. Related: ARVs and PrEP, Nukes

21 December 2025. Related: ARVs and PrEP, Capsid inhibitors, PrEP
(no pic available)
1 April 2013. Related: ARVs and PrEP, CCR5 inhibitors, Entry inhibitors

4 January 2022. Related: ARVs and PrEP, PIs
![]()
No longer recommended in the UK. No longer manufactured.
1 April 2013. Related: ARVs and PrEP, NNRTIs

Nevirapine 200 mg

Nevirapine-PR (prolonged release) 400 mg
4 January 2022. Related: ARVs and PrEP, Fixed dose combinations, NNRTIs, Nukes

Full details about Odefsey (rilpivirine + TAF + emtricitabine)
12 April 2016. Related: ARVs and PrEP, PIs, PK booster

Full details about Prezcobix (darunavir + cobicistat) US name
3 January 2022. Related: ARVs and PrEP, Integrase inhibitors


Once-daily (600mg) Twice-daily (400 mg)
26 October 2016. Related: ARVs and PrEP, PIs, PK booster
12 May 2018. Related: ARVs and PrEP, NNRTIs
![]()
1 April 2013. Related: ARVs and PrEP, PIs, PK booster

New Meltrex tablet (non-refrigerated)
![]()
Old (original) capsule (requires refrigeration)
26 August 2012. Related: ARVs and PrEP, PIs
![]()
4 January 2022. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes
![]()
Full details about Stribild (elvitegravir + cobicistat + emtricitabine (FTC) + tenofovir DF)
12 May 2018. Related: ARVs and PrEP, Fixed dose combinations, Nukes, PIs

Full details about Symtuza (darunavir + cobicistat + emtricitabine + TAF)
26 August 2012. Related: ARVs and PrEP, Entry inhibitors, Fusion inhibitors
![]()
12 February 2018. Related: ARVs and PrEP, Nukes, PrEP
No picture: TAF is only available in coformulations with other HIV drugs.
1 December 2020. Related: ARVs and PrEP, Nukes, PrEP
![]()
26 August 2012. Related: ARVs and PrEP, PIs
![]()
1 January 2025. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, NNRTIs
Picture not included as there are many different versions.
Full details about TLD or LTD (tenofovir disoproxil, lamivudine, dolutegravir)
2 February 2025. Related: ARVs and PrEP, Fixed dose combinations, NNRTIs, Nukes
Please see Atripla.
30 August 2024. Related: ARVs and PrEP, Fixed dose combinations, Integrase inhibitors, Nukes

26 August 2012. Related: ARVs and PrEP, Fixed dose combinations, Nukes
![]()
4 January 2022. Related: ARVs and PrEP, Nukes, PrEP
![]()